Words like “failure” can be scary. In the world of medical laboratory science, the word failure takes on a whole new level of serious, real-life consequences, should failures occur during any kind of testing. Thankfully, when it comes to investigating proficiency testing (PT) failures, there is an abundance of information available online to help clinical laboratories develop corrective and preventative actions to avoid common pt errors.
Pre-examination, examination, and reporting checklists that are generated in root cause analysis, like this handy one from Lablogatory, can serve as a “launch pad” for the development of guidelines to take corrective action. Clinical laboratories use root cause analysis to identify, define, and resolve a core issue, so that resulting errors or failures cease in future proficiency testing efforts. This systematic process of analysis can help us ask the questions that give us the full story of why a failure occurred.
Much like journalists, we laboratory professionals ask “who, what, when, where, and why” questions to help us investigate. In the vein of root-cause analysis, WSLH Proficiency Testing has provided detailed scenarios of common PT failures exemplified throughout the proficiency testing process, from pre-examination to reporting. The review of detailed scenarios in specific proficiency testing programs, which are couched in real-world, empirical evidence can aid clinical lab staff in the development of strategies from investigation to action.
Let’s visit the world of hematology proficiency testing for our first scenario. Since hematology proficiency testing (PT) samples are manufactured material, most PT samples for hematology have to be tested in the quality control (QC) mode instead of patient mode to recover the correct values. In one particular instance, a laboratory did not use the barcode provided on the PT sample. Instead, the lab applied their own barcode which triggered the sample to be tested in patient mode, leading to failures on the differential parameters. The barcode provided by the PT provider would have triggered the sample to be tested in the correct QC mode; and, the lab would have recovered the correct values.
This is a common sample handling error seen in hematology PT and is very avoidable. Many times, labs are instructed to perform remedial action as required by their accreditation agency as a result of this handling error. Sometimes it is helpful to see an example, in order to be able to identify the causes that result in a lab testing in the wrong mode.
Please see the snippet of a 2020 HemeReg1 report, for example.
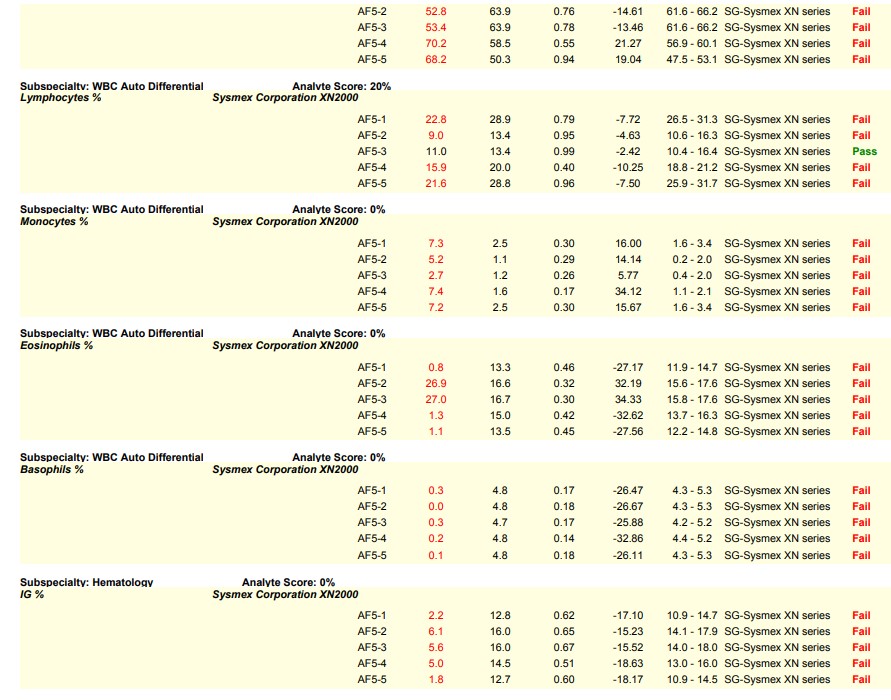
Here are some key steps and resources to consider in avoiding this common error:
- Always read the proficiency testing sample handling instructions that come with your PT kit. In this particular instance, there were specific handling instructions provided for each type of hematology instrument explaining how to test the PT samples in QC mode. If you do not understand the handling instructions, call your PT provider for clarification.
- Your hematology instrument manufacturer can also be a resource to assist you with testing your PT samples in the mode specified by your PT sample handling instructions.
- The CLIA Proficiency Testing and PT Referral booklet (pg. 7) provides some helpful guidance explaining that, although you are required to test PT samples like you would patient samples, sometimes PT samples require special treatment.
In the world of Blood Bank (Immunohematology) proficiency testing, the need for labs to seek out remedial testing most often stems from compatibility failures. The Blood Bank Comprehensive program includes a set of five samples (unknowns), plus a donor cell for compatibility testing. When reporting online, it is important to list the compatibility testing method used for each sample. Select if an immediate spin or anti-human globulin (AHG) method was used, so that the type of testing matches the situation and your lab protocol. Some labs use a combination of immediate spin (negative screen) and AHG (positive screen) testing. Others may use AHG for all compatibility testing samples regardless of the screen result. Let’s take a look at these two common errors and helpful hints we can employ to sidestep these failures in the future:
- If your lab performs anti-human globulin (AHG) crossmatches, you must perform them on the PT survey when warranted (i.e., when a sample has a positive antibody screen). Do not report an immediate spin crossmatch interpretation as your final result on samples that warrant an AHG crossmatch (has a positive screen) or you risk failure.
- Labs that routinely perform both immediate spin and AHG testing on all samples should report the serologic interpretation as “Not compatible” if either test is positive.
- Per CMS, labs using antiglobulin crossmatch methods (automated or manual) that employ a technology only designed to detect incompatibilities due to IgG antibodies, must also use an immediate spin crossmatch to detect incompatibilities due to IgM antibodies (i.e., ABO incompatibilities).
(Please see the snippet of a 2020 BloodBank3 report, for example.)
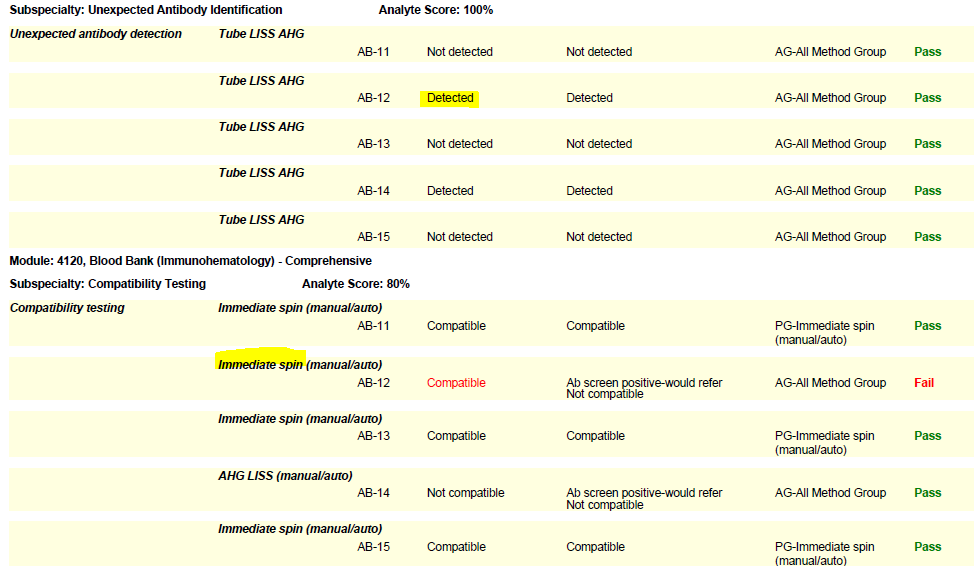
Immunohematology proficiency testing samples have a shorter shelf life, as they are manufactured to simulate whole blood. When the PT event closes, these samples expire. Therefore, if a lab needs to troubleshoot a failure, the age or expiration of the samples should be noted with follow-up documentation.
The oversight of any step, however small, can create major setbacks in proficiency testing. One such oversight commonly made among clinical laboratories is the failure to identify a PT sample—in other words, to treat a proficiency testing sample like a patient sample. Recently, one of our proficiency testing program coordinators received a call from a lab requesting replacement of Blood Gas proficiency testing samples. Upon receiving more context from the lab supervisor, the WSLH PT program coordinator learned that the lab had given the sample set and instructions to the selected Point-Of-Care analyst who was to run proficiency testing for the current event. The analyst ran all five samples without inputting the sample ID into the instrument (i-STAT). They then returned their printouts to the supervisor for submission to WSLH PT. When questioned, they reported that the samples had been run “in order”, but had no proof; so, those results could not be submitted for the event. Subsequently, the supervisor had to call WSLH PT to order (and pay for) a replacement sample set to retest with the sample IDs before they could complete the event.
These detailed, real-world instances that WSLH Proficiency Testing program coordinators have provided aim to assist clinical lab staff in the development of their own strategies, which are situated in root cause analysis. Investigation, planning, and communication with all participating staff can help clinical laboratories get to the root cause of common problems, and to make sure such errors do not happen again. WSLH Proficiency Testing offers a corrective action form online for clinical laboratories to use as a tool or guideline for investigating PT failures. Please refer to our Resource page where you can find this form among other helpful resources for clinical laboratories conducting PT. Utilizing resources and learning from scenarios such as these can aid laboratory professionals in initiating conversations, investigations, and trainings with staff so that common PT failures may be avoided in the future.








 By Kristine Hansbery
By Kristine Hansbery